MDMRCI and ARMCI Cluster Research Ethics Review Committee
The Metro Davao Medical and Research Center Inc. and the Anda River View Medical Center Inc. (MDMRCI-ARMCI CRERC) was created on January 5, 2015. It is an independent body created by the consortium between Metro Davao Medical and Research Center Inc. and Anda River View Medical Center Inc. under each Hospital Director, who was appointed by the President of MDMRCI, and President/Vice President of ARMCI respectively. It aims to protect human participants and contribute to the highest attainable quality of scientific and ethical research.
MDMRCI
MDMRCI
will be the most preferred family-centered health & wellness institution by
clients and their families and healthcare professionals in Mindanao by 2020
We are
a family-centered health and wellness institution, composed of competent health
care professionals, promoting innovative research, collaborative health
advocacies, utilizing state of the art technology in a safe, comfortable, and
pleasant healing environment.
ARMCI
A leading healthcare facility with excellent, affordable and people-friendly services provided by dedicated and fulfilled members, blending the best appropriate technology in a caring and humane environment.
To serve the healthcare needs of the people of Davao and its neighboring environment through excellent customer-responsive and affordable services.
Organizational Chart
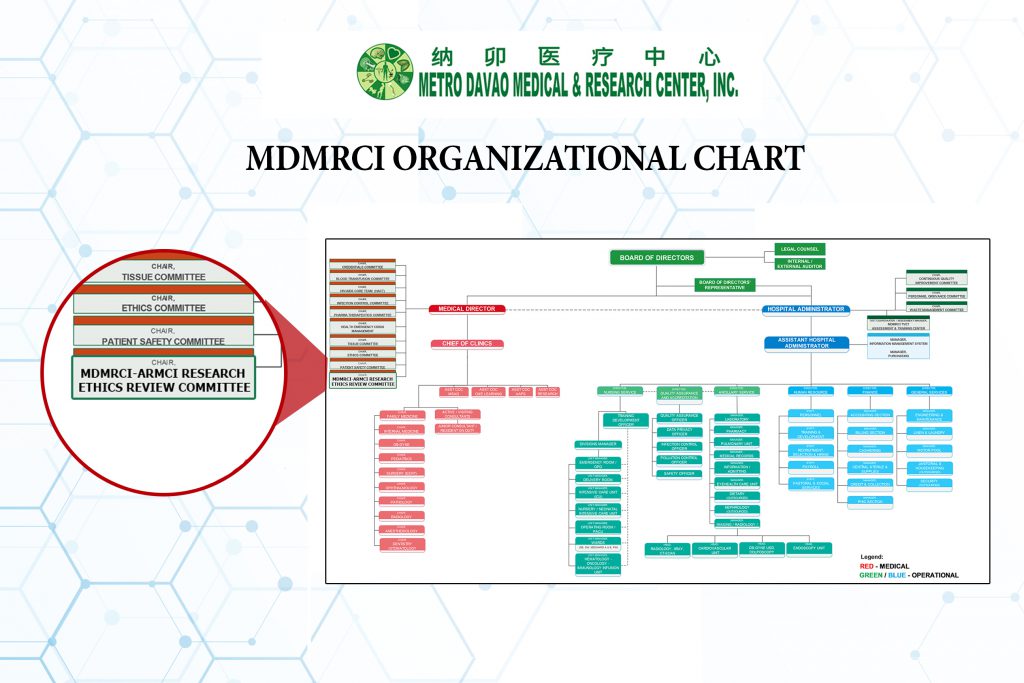
MDMRCI ORGANIZATIONAL CHART
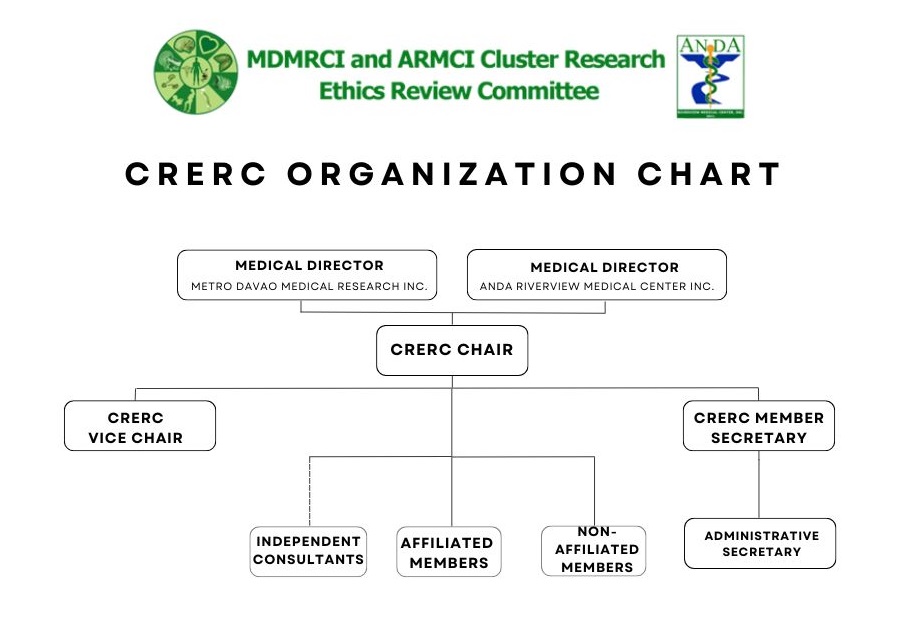
CRERC ORGANIZATIONAL CHART
MEMBERS OF CRERC
Affiliated Members
- Annabelle Lao-Reyes, MD – (Neurology) Affiliated, Medical – Chair
- Arthur Gregory A. Lui, MD -(Oncology) Affiliated, Medical – Vice Chair
- Jose Paolo P. Panuda, MD – (Endocrinology) Affiliated, Medical
- Deborah Juliet A. Omran, RN, MAN, PhD – (Nurse) Affiliated, Non-Medical
Non-Affiliated Members
- Milagros M. Viacrucis, MD – (Pediatrics and Public Health) Non-affiliated, Medical – Secretary
- Adorico A. Aya-ay, PhD – (Teacher) Non-affiliated, Non-medical & Scienctist
- David A. Tan –(Pastor) Non-affiliated, Lay/Community Representative
List of Independent Consultants:
- Vanina Htun-Javier, MD – Oncologist
- Julie Ann Kristy L. Torres, MD – Neurologist
- Bermillion Faderan, MD – Cardiologist
- Jonray R. Magallanes, MD – Pulmonologist
- Jennifer Ivy Ortega Togonon-Leaño, MD – Nephrologist
INITIAL REVIEW

POST-APPROVAL REVIEW

FOR SUBMISSION OF INITIAL REVIEW APPLICATION AS WELL AS SUBMISSION OF PROTOCOL AMENDMENT AND ANNUAL PROGRESS REPORT PROTOCOL PACKAGE
1. Submit Initial Review Application through email: MDMRCI-ARMCI CRERC [email protected]
2. SUBJECT of the email should be: INITIAL SUBMISSION/Name of Principal Investigator (PI)
3. For e-mail content: Please refer to CRERC Form 3.4 Document Submission Checklist
4. All documents shall be signed and dated as indicated.
5. Incomplete submission will not be accepted.
6. Submission of print/paper copy of the docucment.
COMMUNICATION:
PLease address all communication to:
ANNABELLE LAO-REYES, MD, MDMRCI-ARMCI CRERC Chairperson
BOARD MEETING:
Board meeting is scheduled every first Tuesday of the month. Cut-off date for submission (Initial Submission, Resubmission, Amendment, Continuing Review Application, Protocol Deviation Report, and SAE Report, and Notification from the PI) is 15 calendar days before the board meeting.
Special Meeting may be conducted for facilitated review of public health emergency protocols.
STATEMENT OF ACCOUNT (REVIEW FEES)
Submit the following information:
● Sponsor Code (If applicable)
● Name of Researcher or Principal Investigator
● Sponsor:
● Bill to:
● Billing Address:
CRERC will schedule the review once payment of the initial review fee has been settled with the Metro Davao medical & Research Center, Inc. cashier. (Submit photocopy of OR to the CRERC Administrative Secretary).
Schedule of CRERC Meeting: 1st Tuesday of the month
Deadline for Submission of Document Package for Review:
Deadline for Submission of Document Package for Review:
Initial Review of study protocols of more than minimal risk or involve vulnerable population | at least 4 weeks before the date of CRERC meeting |
Major Protocol Amendment | at least 2 weeks before the date of CRERC meeting |
Protocol-related submission for continuing review | at least 2 weeks before the date of CRERC meeting |
Early Study Termination Report (with study subjects randomized to treatment) | at least 2 weeks before the date of CRERC meeting |
SAE Report, Protocol Deviation Report, Final Report, Early Study Termination Report where no subjects were randomized | At least 1 week before the date of CRERC meeting agenda does not include a protocol for initial review |
Required Documents for Review:
List of downloadable SOPs and Forms
FORMS
- CRERC Form 3.1 Application for Initial Review
- CRERC Form 3.2 Protocol Summary Sheet
- CRERC Form 3.3 Protocol Format Checklist
- CRERC Form 3.4 Document Submission Checklist
- CRERC Form 3.5 Study Site Resources Checklist
- CRERC Form 3.6 Declaration-of-Conflict-Of-Interest Study Site Resources Checklist
- CRERC Form 4.3 Protocol Assessment Checklist
- CRERC Form 4.4 Informed Consent Assessment Checklist
- CRERC Form 4.5 Checklist for Exemption from Ethical Review
- CRERC Form 4.6 Protocol Resubmission
- CRERC Form 7.1 Application for Protocol Amendment
- CRERC Form 7.3 Progress/Annual Report
- CRERC Form 7.4 Final Report
- CRERC Form 7.5 Onsite Serious Adverse Event Report
- CRERC Form 7.6 Protocol Violation/Deviation Report
- CRERC Form 7.7 Early Termination of the Study Report
- CRERC Form 11.1 Query/Complaint, or Notice
REFERENCES
CRERC Review Meetings and Cut-off Date of Submission
a.) Schedule of regular meeting - First Tuesday of the Month
b.) Correction on cut-off dates (15 Days before date of meeting)
c.) Submission of Protocol for Initial Review
Location
Km. 4 JP Laurel Ave. Bajada Davao City
Contact us
Email: [email protected]
Phone: (082) 287-7777 Local 8103
Office Hours: 8:00 AM to 5:00 PM (Monday to Friday)
